The Chemistry of Honey
The Chemistry of Honey
Have you ever wondered what honey is made up of?
Here is a simple explanation of the chemistry of honey:
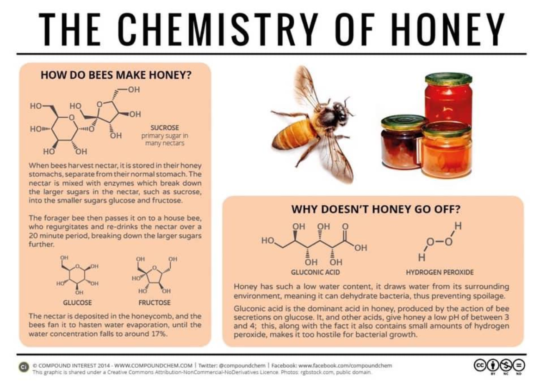
When bees collect nectar they store it in their honey stomachs. (This is separate from their normal stomach. ) Inside the honey stomach, nectar mixes with enzymes thus breaking down the large sucrose sugars into fructose and glucose.
When the forager bee returns to the hive, it passes the nectar on to a house bee who regurgitates and re-drinks the nectar breaking the sugars down further. Next step is to deposit the nectar in the honeycomb where the bees fan it to hasten water evaporation. Once the water concentration falls to around 17% the honey is capped with wax for permanent storage.See link to: https://www.hiveworld.co.nz/keeping-your-honey-part-1/
Because of this low water content, honey draws water from its environment. This means it can dehydrate bacteria, thus preventing spoilage. Due to the gluconic acid in honey (produced by the action of bee secretions on glucose) along with other acids, it has a low pH of between 3 and 4. As well as this, honey contains small amounts of hydrogen peroxide thereby making it too hostile for bacteria to grow. (The secret of honey’s healing powers?)

 The Chemistry of Honey
The Chemistry of Honey